- 产品详情
- 说明书下载
- 引用文献
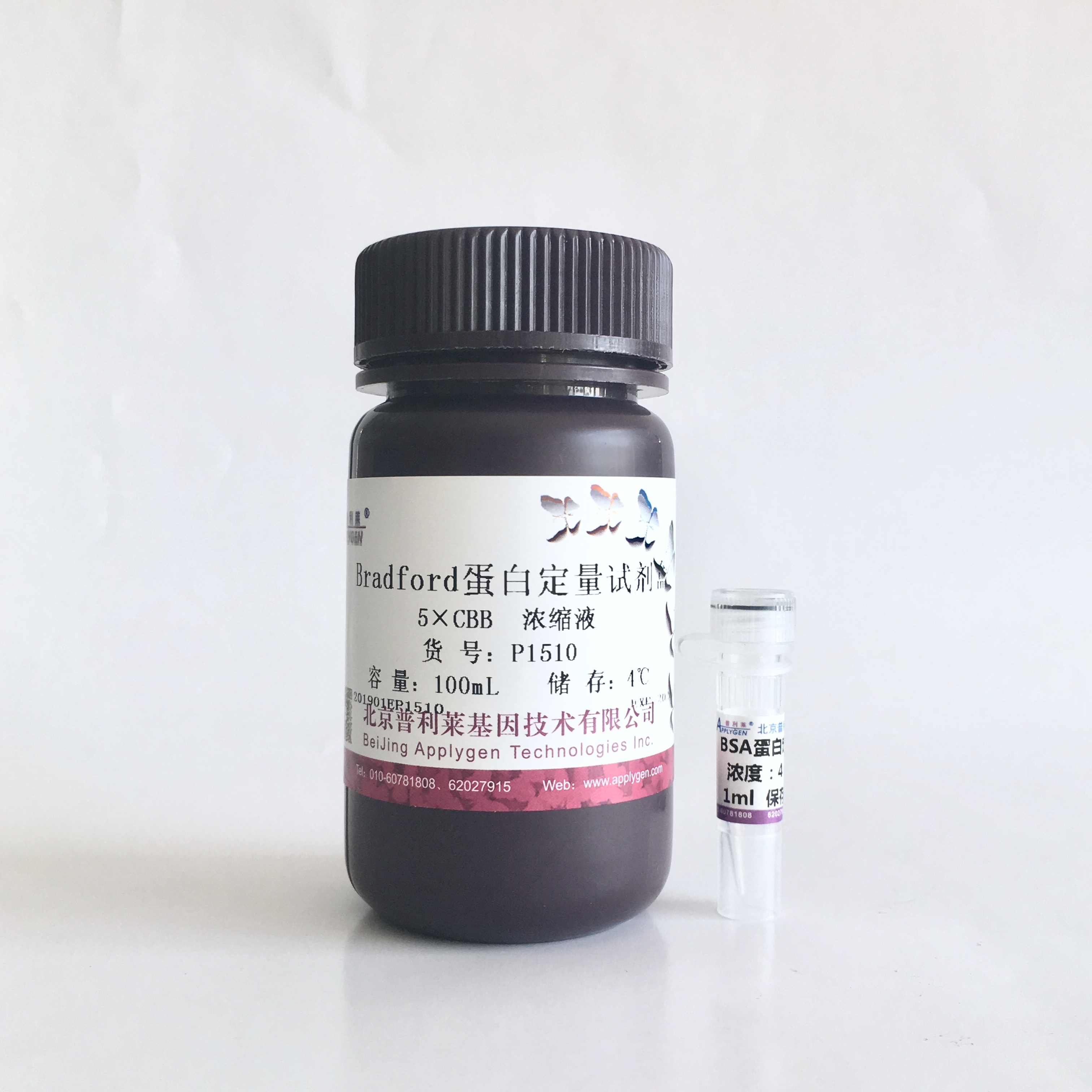
描述:考马斯亮蓝蛋白定量法又称Bradford法,是最常用的蛋白质快速定量方法之一。考马斯亮蓝(Coomassie brilliant blue G-250)与蛋白质结合后染料的最大吸收峰由465nm变为595nm,溶液的颜色由棕色变为蓝色,在595nm波长下测定的吸光度值与蛋白含量成正比。灵敏度比Lowry法高4倍,与BCA法相当。反应迅速,2分钟达到平衡并在1小时内保持稳定。操作简便,只需要一种反应试剂。干扰物质少,K+、Na+、Mg2+离子、Tris、葡萄糖和蔗糖、甘油、巯基乙醇、(NH4)2SO4、乙醇、EDTA等均不干扰此测定法。本试剂盒采用改良Bradford法和优化的试剂组成以及优化的测定方案,可对0.5-4000µg/ml浓度范围的蛋白进行线性检测,显著提高了蛋白检测灵敏度。提供的5 ´ 染料浓缩液可进行400次标准2ml比色杯检测,或2666次微板检测。
组成与储存: 1) 5´Coomassie浓缩溶液 100ml 室温或4ºC储存
2) 牛血清白蛋白标准品(BSA) 4.0mg/ml溶液 1ml −20ºC储存
所需设备:比色计或酶标仪、微板比色仪,工作波长为595nm±5nm (见说明1)。
操作步骤:
一、工作染液配制
用蒸馏水将5×Coomassie浓缩液稀释为500ml 1×染料工作液。稀释的1×染料工作液用普通滤纸过滤除去沉淀,否则可能无法使用。1×染料工作液在4ºC避光保存9个月,放置后如由茶色变蓝色可再次过滤。加入反应管前应升到室温,直接使用4ºC工作液不影响精度但测得的OD值较低。
二、标准蛋白溶液配制
按照表1和下页说明将标准牛血清白蛋白(BSA,4mg/ml,-20ºC)稀释为需要的浓度。可用双蒸水、0.9%生理盐水、PBS缓冲液或待测蛋白样品的缓冲液稀释。
三、标准曲线制作
取8支干净试管,按下表加样。将试管中溶液充分混匀,放置2min。将溶液分别加入1 cm光径的比色皿(或取200µl加入96孔微板),用分光光度计、酶标仪、微板测量仪测定595 nm处的光吸收值OD595。用标准蛋白质浓度(mg/ml)为横坐标,用吸光度值OD595为纵坐标作图,即得标准曲线。
样品测定与浓度计算
样品测定方法同上(标准曲线制作)。根据标准曲线和测出的未知样品的OD595值,查出未知样品的蛋白质浓度。此浓度是与染料工作液混合之前的待测样品中的蛋白浓度。如果样品在与染料工作液混合之前经过稀释,最后估计样品蛋白浓度时须乘以实际稀释倍数。
表1 几种不同测定方案的加样量和比例
|
测定方案 |
超敏感方案 |
高敏感方案 |
标准方案 |
|||
|
测定范围 |
0.5-50 µg/ml |
5-500 µg/ml |
50-2000 µg/ml |
|||
|
微量测定或标准测定 |
微量 |
标准 |
微量 |
标准 |
微量 |
标准 |
|
检测管数 |
2666 |
400 |
1400 |
210 |
1400 |
200 |
|
加入标准蛋白或待测蛋白样品溶液 µl |
150 |
1000 |
15 |
100 |
3 |
20 |
|
精确加入考马斯亮蓝染料工作液 µl 或者为简便操作加入以下近似量µl |
150
|
1000 |
285 300 |
1900 2000 |
297 300 |
1980 2000 |
|
反应总体积 µl |
300 |
2000 |
300 |
2000 |
300 |
2000 |
|
标准或待测蛋白样品与考蓝工作液的比例 |
1:1 |
1:1 |
1:20 |
1:20 |
1:100 |
1:100 |
标准测定:反应终体积2ml。用1cm光程玻璃或塑料比色皿,用比色计测定。
微量测定:反应终体积300µl。用96孔微板,用酶标仪、微板比色仪测定。
标准方案(50-2000 µg/ml)标准曲线的标准蛋白倍比稀释:
标准测定时:30µl 4000µg/ml BSA + 30µl稀释溶液(H2O/PBS/0.9%NaCl) = 60µl (BSA=2000µg/ml),取30µl连续倍比稀释7次,得到BSA标准溶液2000、1000、500、250、125、62.5、31.25、15.625µg/ml。每一稀释度标准溶液各取20µl,与1980ml或者2000ml染料工作液混合。
微量测定时:5µl 4000µg/ml BSA + 5µl稀释溶液(H2O/PBS/0.9%NaCl) = 10µl (BSA=2000µg/ml),取5µl连续倍比稀释7次。得到BSA标准溶液2000、1000、500、250、125、62.5、31.25、15.625 µg/ml。每一稀释度标准溶液各取3µl,与297或300 ml蓝染料工作液混合。
高敏感方案(5-500µg/ml)标准曲线的标准蛋白倍比稀释:
标准测定时:37.5µl 4000 µg/ml BSA + 262.5µl稀释溶液(H2O/PBS/0.9%NaCl) = 300µl (BSA=500µg/ml),取150µl连续倍比稀释7次。得到BSA标准溶液500、250、125、62.5、31.25、15.625 、7.813、3.907µg/ml。每一稀释度标准溶液各取100 µl,与1800ml或者2000ml染料工作液混合。
微量测定时:3.75µl 4000µg/ml BSA + 26.25µl稀释溶液(H2O/PBS/0.9%NaCl) = 30µl (BSA=500µg/ml),取15µl连续倍比稀释7次。得到BSA标准溶液500、250、125、62.5、31.25、15.625 、7.813、3.907µg/ml。每一稀释度标准溶液各取15µl,与285ml或者300ml染料工作液混合。
超敏感方案(0.5-50µg/ml)标准曲线的标准蛋白倍比稀释
标准测定时:37.5µl 4000µg/ml BSA + 2962.5 µl稀释溶液(H2O/PBS/0.9%NaCl) = 2000µl (BSA=50µg/ml),取1000µl连续倍比稀释7次。得到BSA标准溶液50、25、12.5、6.25、3.125、1.563、0.782、0.391 µg/ml。每一稀释度标准溶液各取1000 µl,与1000ml染料工作液混合。
微量测定时:3.75µl 4000µg/ml BSA + 296.25µl稀释溶液(H2O/PBS/0.9%NaCl) = 300µl (BSA=50µg/ml),取150µl连续倍比稀释7次。得到BSA标准溶液50、25、12.5、6.25、3.125、1.563、0.782、0.391 µg/ml。每一稀释度标准溶液各取150µl,与150ml染料工作液混合。
注意事项
1. 分别在工作波长为595nm和450nm测定OD值,计算OD594/OD450,以此代替原有的OD595作图,可明显提高检测的精确度和灵敏度约10倍,显著提高测定的线性关系,并降低去垢剂的影响。在进行超敏感度测定时可考虑此法。
2. 主要干扰物质的终浓度:SDS<0.002%;Triton X-100<0.1%;Tween-20/60/80<0.015%;NaOH<0.1N。
3. 蛋白-染料结合物在1小时内保持稳定,但在5-20分钟内稳定性最好,因此应尽快测定。如果放置过久将出现沉淀,或使测定结果偏低。
4. 染料不结合精氨酸、赖氨酸及分子量小于3kDa的短肽,所以不同的蛋白质由于精氨酸和赖氨酸含量不同会出现小范围的测量偏差;小于3kDa的短肽纯品不能用此方法测定。
5. 染料-蛋白结合物附着在石英比色皿后难以洗去,因此不宜使用石英比色皿。可用玻璃或塑料比色皿,用后立即用95%乙醇或洗涤剂浸泡洗涤。塑料比色皿不可用乙醇长时间浸泡。
6. 标准曲线应该每次新做。但每一稀释度的BSA标准品可预先配好-20ºC冻存备用,注意在使用这种非新鲜配制的BSA时,如果发现标准曲线的某一些点偏差较大,说明稀释的BSA已经坏掉应予重配。
7. Bradford法物质干扰及耐受的最大浓度参见公司网站pdf文件。
目前,使用普利莱考蓝法蛋白定量试剂盒发表的SCI文章已达数百篇,部分列举请参考。
1. Qiu L Z, Zhou W, Yue L X, et al. Repeated Aconitine Treatment Induced the Remodeling of Mitochondrial Function via AMPK–OPA1–ATP5A1 Pathway[J]. Frontiers in Pharmacology, 2021, 12: 646121.
2. Yan Y, Zhou Y, Li J, et al. Sulforaphane downregulated fatty acid synthase and inhibited microtubule-mediated mitophagy leading to apoptosis[J]. Cell Death & Disease, 2021, 12(10): 917.
3. Xie S Y, Wang S L, Zhao B K, et al. Effect of PLGA as a polymeric emulsifier on preparation of hydrophilic protein-loaded solid lipid nanoparticles[J]. Colloids and Surfaces B: Biointerfaces, 2008, 67(2): 199-204.
4. Xiong H, Wei L, Peng B. IL‐17 stimulates the production of the inflammatory chemokines IL‐6 and IL‐8 in human dental pulp fibroblasts[J]. International endodontic journal, 2015, 48(6): 505-511.
5. Zhu L, Wei H, Wu Y, et al. Licorice isoliquiritigenin suppresses RANKL-induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo[J]. The international journal of biochemistry & cell biology, 2012, 44(7): 1139-1152.
6. Feng Y, Yin J, Jiao Z, et al. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats[J]. Toxicology letters, 2012, 211(2): 201-209.
7. Feng Y, Sun F, Gao Y, et al. Taurine decreased uric acid levels in hyperuricemic rats and alleviated kidney injury[J]. Biochemical and biophysical research communications, 2017, 489(3): 312-318.
8. Xie S, Wang S, Zhu L, et al. The effect of glycolic acid monomer ratio on the emulsifying activity of PLGA in preparation of protein-loaded SLN[J]. Colloids and Surfaces B: Biointerfaces, 2009, 74(1): 358-361.
9. Wei L, Liu M, Xiong H, et al. Up‐regulation of IL‐23 expression in human dental pulp fibroblasts by IL‐17 via activation of the NF‐κB and MAPK pathways[J]. International endodontic journal, 2018, 51(6): 622-631.
10. Wang Y, Zhang J, Zhu L, et al. Positive correlation between activated CypA/CD147 signaling and MMP-9 expression in mice inflammatory periapical lesion[J]. BioMed Research International, 2019, 2019.
11. Feng Y, Lin S, Zhao X, et al. Taurine Inhibited Uric Acid Uptake in HK-2 Renal Tubular Epithelial Cells[C]//Taurine 11. Springer Singapore, 2019: 147-154.
12. Sun H, Che Q M, Zhao X, et al. Antifibrotic effects of chronic baicalein administration in a CCl4 liver fibrosis model in rats[J]. European journal of pharmacology, 2010, 631(1-3): 53-60.
13. Zhang H, Xu C F, Ren C, et al. Novel role of p53 in septic immunosuppression: involvement in loss and dysfunction of CD4+ T lymphocytes[J]. Cellular Physiology and Biochemistry, 2018, 51(1): 452-469.
14. Feng Y, Shi Z, Fang X, et al. Perfluorononanoic acid induces apoptosis involving the Fas death receptor signaling pathway in rat testis[J]. Toxicology letters, 2009, 190(2): 224-230.
15. Zhu L, Wu Y, Wei H, et al. Up-regulation of IL-23 p19 expression in human periodontal ligament fibroblasts by IL-1β via concurrent activation of the NF-κB and MAPKs/AP-1 pathways[J]. Cytokine, 2012, 60(1): 171-178.
16. Wang S L, Xie S Y, Zhu L Y, et al. Effects of poly (lactic-co-glycolic acid) as a co-emulsifier on the preparation and hypoglycaemic activity of insulin-loaded solid lipid nanoparticles[J]. IET nanobiotechnology, 2009, 3(4): 103-108.
17. Ren L, Yang Z, Song J, et al. Involvement of p38 MAPK pathway in low intensity pulsed ultrasound induced osteogenic differentiation of human periodontal ligament cells[J]. Ultrasonics, 2013, 53(3): 686-690.
18. Huang H, Zhao X, Chen Y, et al. Apoptosis induced by ZnPcH1-based photodynamic therapy in Jurkat cells and HEL cells[J]. International journal of hematology, 2011, 94: 539-544.
19. Qin T, Wu L, Hua Q, et al. Prediction of the mechanisms of action of Shenkang in chronic kidney disease: a network pharmacology study and experimental validation[J]. Journal of ethnopharmacology, 2020, 246: 112128.
20. Shi Z, Ding L, Zhang H, et al. Chronic exposure to perfluorododecanoic acid disrupts testicular steroidogenesis and the expression of related genes in male rats[J]. Toxicology letters, 2009, 188(3): 192-200.
21. Yu F Y, Hong Y Y, Qu J F, et al. The large intracellular loop of ptch1 mediates the non-canonical Hedgehog pathway through cyclin B1 in nevoid basal cell carcinoma syndrome[J]. International Journal of Molecular Medicine, 2014, 34(2): 507-512.
22. SalumMchenga S S, Wang D, Janneh F M, et al. Differential dose effects of recombinant IL-25 on the development of dextran sulfate sodium-induced colitis[J]. Inflammation research, 2010, 59: 879-887.
23. Qiao H, Tong Y, Han H, et al. A novel therapeutic regimen for hepatic fibrosis using the combination of mesenchymal stem cells and baicalin[J]. Die Pharmazie-An International Journal of Pharmaceutical Sciences, 2011, 66(1): 37-43.
24. Zhao Z P, Yan F, Ji W H, et al. Identification of immunoreactive proteins of Brucella melitensis by immunoproteomics[J]. Science China Life Sciences, 2011, 54: 880-887.
25. Sun Z, Wang L, Peng B. Kinetics of glycogen synthase kinase (GSK) 3β and phosphorylated GSK 3β (Ser 9) expression in experimentally induced periapical lesions[J]. International Endodontic Journal, 2014, 47(12): 1107-1116.
26. Qin T, Wu Y, Liu T, et al. Effect of Shenkang on renal fibrosis and activation of renal interstitial fibroblasts through the JAK2/STAT3 pathway[J]. BMC Complementary Medicine and Therapies, 2021, 21(1): 1-12.
27. Zhang J, Zhu L, Yan P, et al. Effect of BioAggregate on Receptor Activator of Nuclear Factor-Kappa B Ligand–induced Osteoclastogenesis from Murine Macrophage Cell Line In Vitro[J]. Journal of endodontics, 2015, 41(8): 1265-1271.
28. Zhao G, Yao Y, Lu Z, et al. Up-regulation of mitofusin-2 protects CD4+ T cells from HMGB1-mediated immune dysfunction partly through Ca2+-NFAT signaling pathway[J]. Cytokine, 2012, 59(1): 79-85.
29. Zhong T, Chen J, Ling Y, et al. Down-regulation of neuropathy target esterase in preeclampsia placenta inhibits human trophoblast cell invasion via modulating MMP-9 levels[J]. Cellular Physiology and Biochemistry, 2018, 45(3): 1013-1022.
30. Wu Y, Zhu L, Liu L, et al. Interleukin‐17A stimulates migration of periodontal ligament fibroblasts via p38 MAPK/NF‐κB‐dependent MMP‐1 expression[J]. Journal of cellular physiology, 2014, 229(3): 292-299.
31. Zhang H, Han G, Liu H, et al. The development of classically and alternatively activated macrophages has different effects on the varied stages of radiation-induced pulmonary injury in mice[J]. Journal of radiation research, 2011, 52(6): 717-726.
相关产品

 微信登录
微信登录 收藏本站
收藏本站